Tildrakizumab in Patients with Moderate-to-Severe Psoriasis: Post-Hoc Analyses on Efficacy, Time to Relapse, Long-Term Safety in Elderly Population and Predictability From The reSURFACE 1 and reSURFACE 2 Phase III Clinical Trials -
Real-world efficacy of biological agents in moderate-to-severe plaque psoriasis: An analysis of 75 patients in Taiwan | PLOS ONE
View of Real-life Effectiveness and Safety of Risankizumab in Moderate-to-severe Plaque Psoriasis: A 40-week Multicentric Retrospective Study | Acta Dermato-Venereologica

Secukinumab demonstrates high efficacy and a favorable safety profile over 52 weeks in Chinese patients with moderate to severe plaque psoriasis | Chinese Medical Journal

Figure 3 | Exposure–Response Relationships for the Efficacy and Safety of Risankizumab in Japanese Subjects with Psoriasis | SpringerLink

Absolute Versus Relative Psoriasis Area and Severity Index in Clinical Practice | Actas Dermo-Sifiliográficas

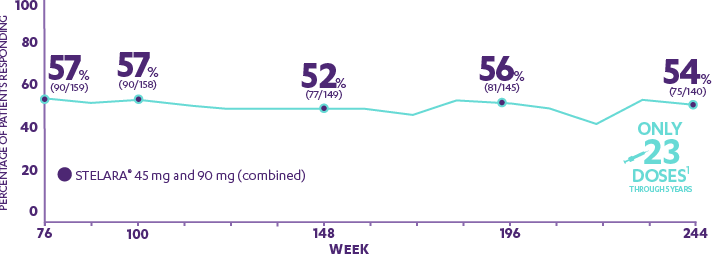
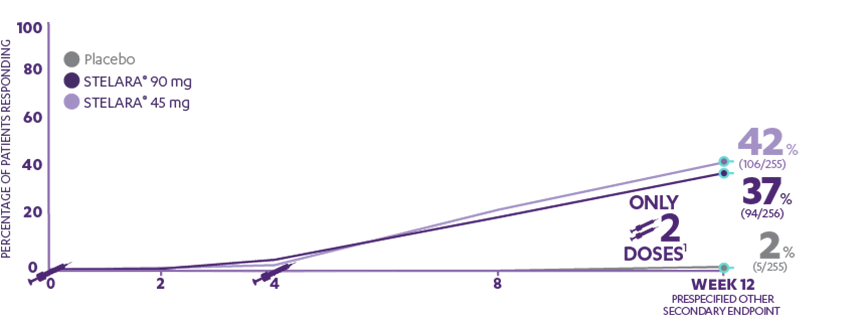
AB0544 EFFICACY AND SAFETY OF TILDRAKIZUMAB IN PATIENTS WITH AND WITHOUT METABOLIC SYNDROME: 5-YEAR POOLED DATA FROM reSURFACE 1 AND reSURFACE 2 | Annals of the Rheumatic Diseases

Clinical response after guselkumab treatment among adalimumab PASI 90 nonresponders: Results from the VOYAGE 1 and 2 trials | Semantic Scholar
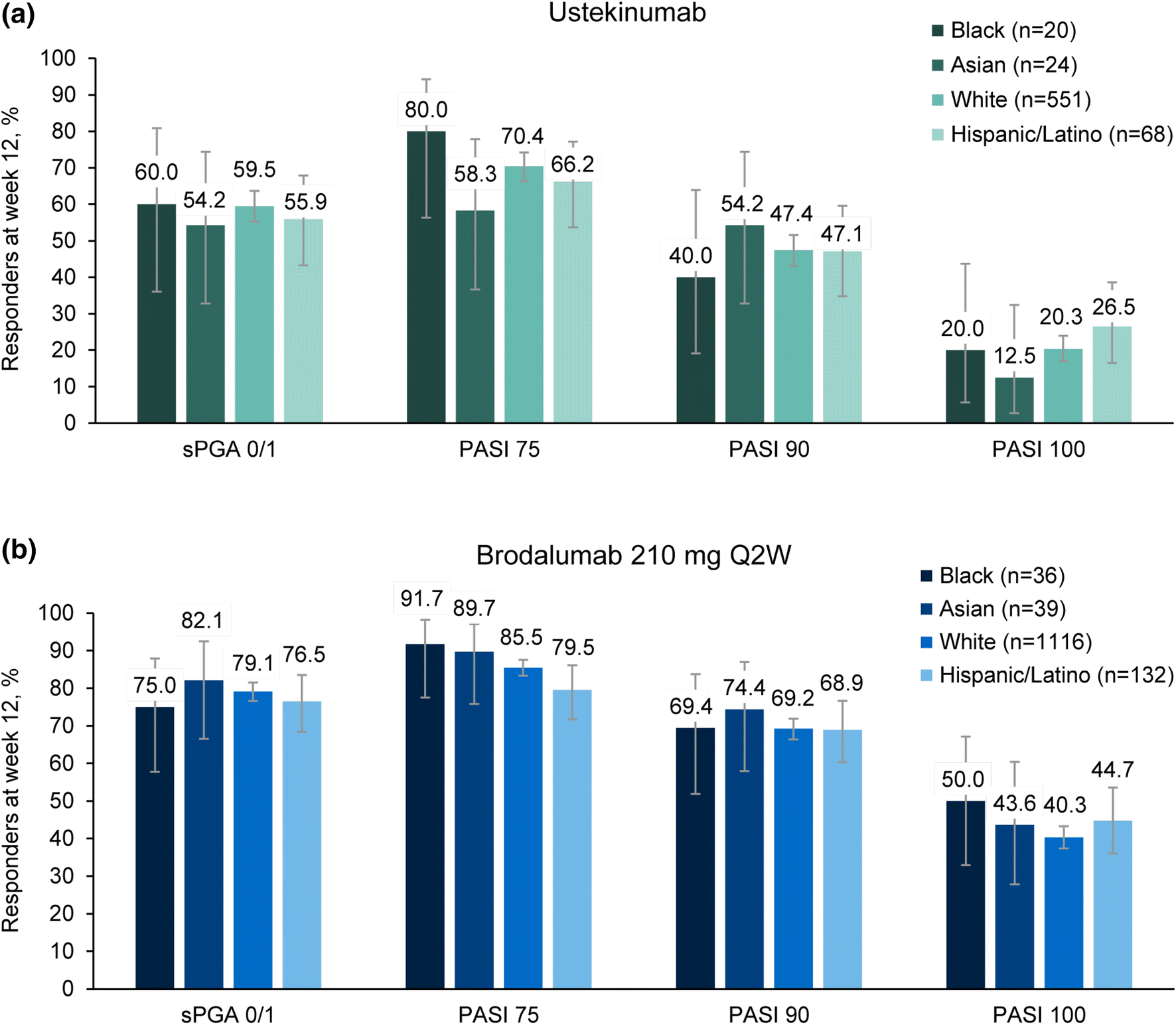
Figure 2 | Efficacy and Safety of Brodalumab in Patients with Moderate-to-Severe Plaque Psoriasis and Skin of Color: Results from the Pooled AMAGINE-2/-3 Randomized Trials | SpringerLink
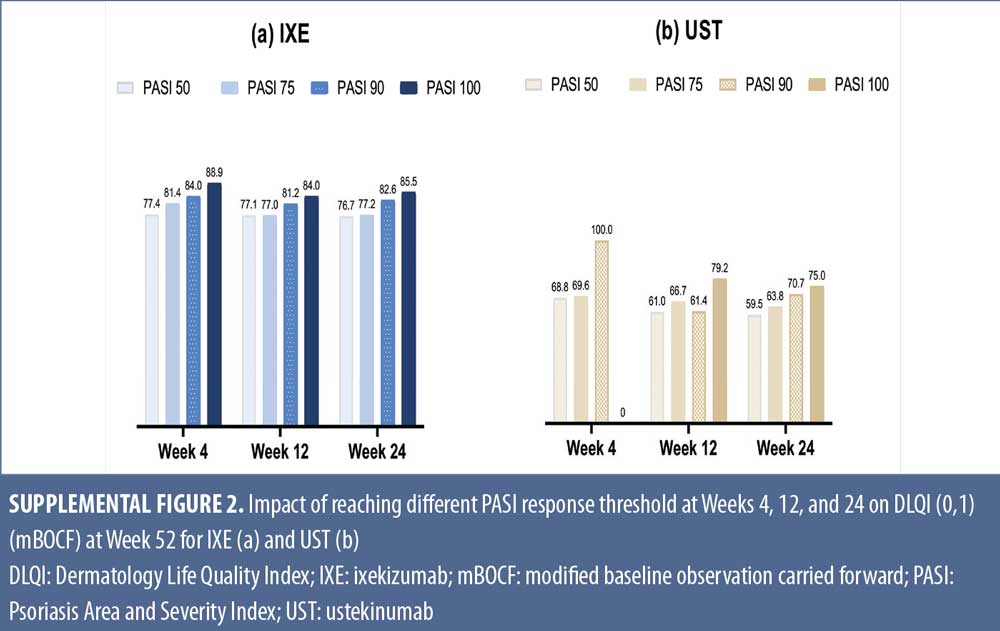
Early Treatment Targets for Predicting Long-term Dermatology Life Quality Index Response in Patients with Moderate-to-Severe Plaque Psoriasis: A Post-hoc Analysis from a Long-term Clinical Study – JCAD | The Journal of Clinical

Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52‐week, open‐label study - Yamasaki - 2017 - British Journal of Dermatology - Wiley Online Library
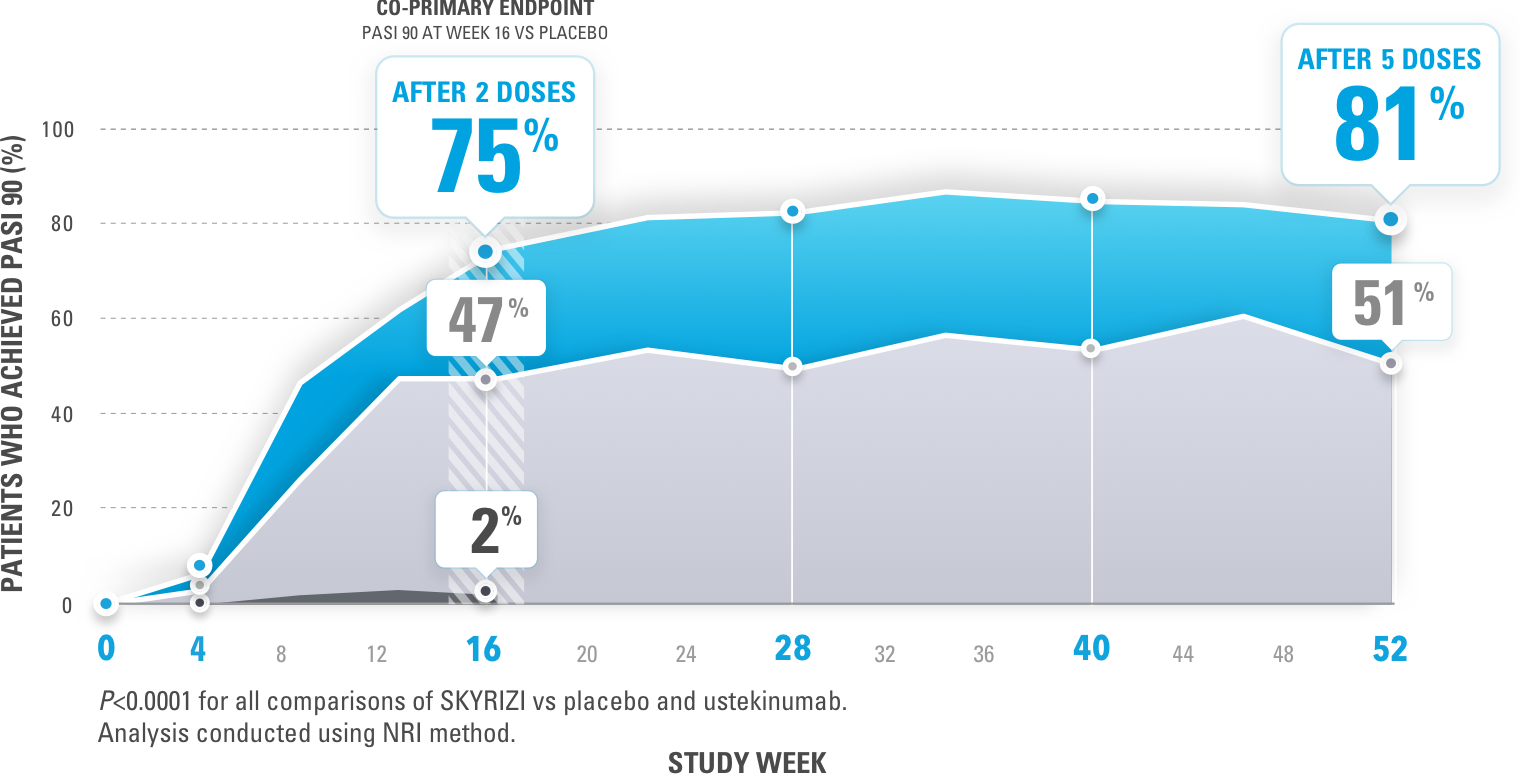
New Novartis data shows Cosentyx™ is significantly superior to Stelara® and clears skin (PASI 90) in nearly 80% of psoriasis patients | Novartis